For US Healthcare Professionals only
PRESCRIBINGINFORMATION REQUEST
SAMPLES

NUZYRA is indicated for the treatment of Community-Acquired Bacterial Pneumonia (CABP) and Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in adults caused by select susceptible microorganisms.

EARLY AND EFFECTIVE CLINICAL SUCCESS IN CABP
OPTIC study design (N=774)1,2
- OPTIC, known as Trial 1 in the NUZYRA Prescribing Information, was a randomized, multinational, double-blind, double-dummy trial comparing the noninferiority of NUZYRA vs moxifloxacin
- The trial compared NUZYRA 100 mg intravenously every 12 hours for 2 doses on Day 1, followed by 100 mg intravenously, or 300 mg orally, daily to moxifloxacin 400 mg intravenously or orally daily in the treatment of adults with CABP. Patients could not switch to oral therapy until Day 4. Total treatment duration was 7 to 14 days
Clinical success in CABP
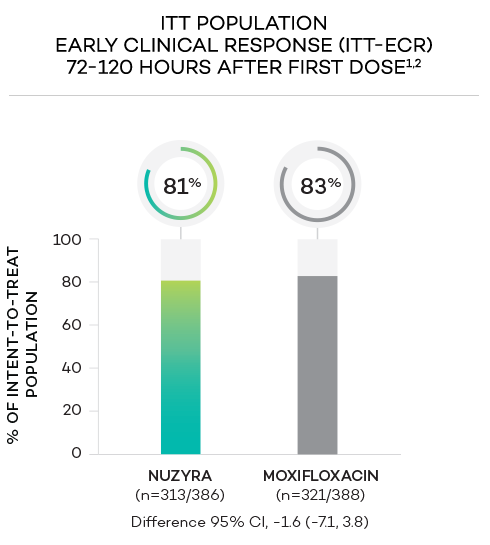
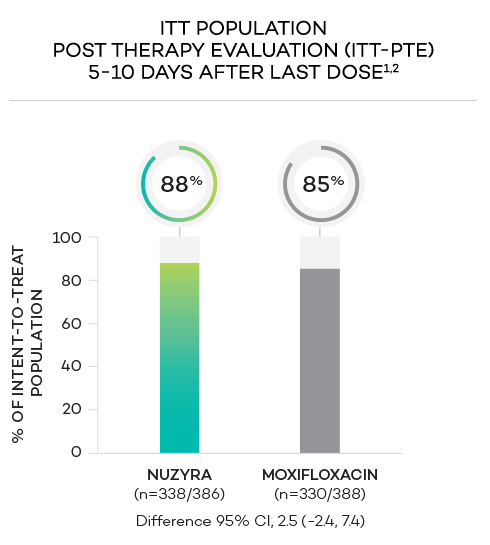
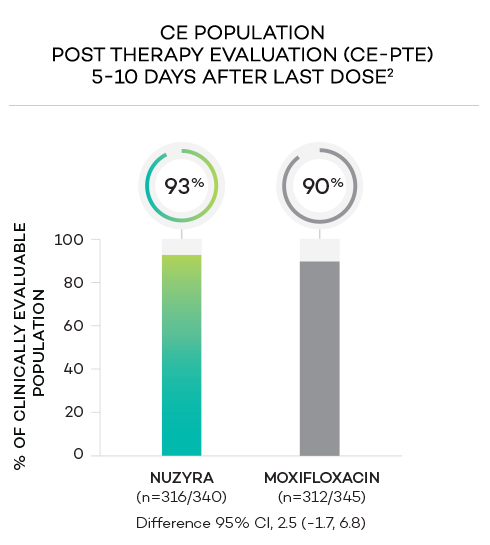
ENDPOINTS1,2
- Primary endpoint at ECR (early clinical response, 72 to 120 hours after the first dose) was defined as survival with improvement in ≥2 of 4 symptoms (cough, sputum production, chest pain, dyspnea) without deterioration in any of these 4 symptoms, and without receiving rescue antibacterial treatment. The primary endpoint was evaluated in the ITT population
- Secondary endpoint at PTE (post therapy evaluation, 5 to 10 days after last dose) was defined as survival and improvement in signs and symptoms of CABP, based on the clinician’s judgment, to the extent that further antibacterial therapy is not necessary, and the infection was considered clinically cured. The secondary endpoint was evaluated in the ITT and in the CE populations
STUDY POPULATIONS1,2
- Intent-to-treat (ITT) population was defined as all randomized patients
- Clinically evaluable (CE) population was defined as all ITT patients who met inclusion criteria and completed the trial
- Microbiological ITT (micro-ITT) population was defined as all randomized patients who had a causative pathogen at baseline
CLINICAL SUCCESS RATES BY PATHOGEN AT PTE IN CABP1,a
| PATHOGEN | NUZYRA
% (n/N) |
MOXIFLOXACIN
% (n/N) |
|---|---|---|
| Streptococcus pneumoniae | 86% (37/43) | 91% (31/34) |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 73% (8/11) | 80% (8/10) |
| Haemophilus influenzae | 81% (26/32) | 100% (16/16) |
| Haemophilus parainfluenzae | 83% (15/18) | 77% (13/17) |
| Klebsiella pneumoniae | 77% (10/13) | 85% (11/13) |
| Legionella pneumophila | 93% (27/29) | 96% (27/28) |
| Mycoplasma pneumoniae | 89% (31/35) | 86% (25/29) |
| Chlamydophila pneumoniae | 93% (14/15) | 93% (13/14) |
aInvestigator’s overall assessment of clinical response at PTE by baseline pathogen in OPTIC (micro-ITT population).
Safety data for NUZYRA
in the treatment of CABP
Is NUZYRA covered by Commercial
and Medicare insurance plans?
Considering NUZYRA
for your patients?
Expand
Collapse
INDICATIONS and IMPORTANT SAFETY INFORMATION
INDICATIONSNUZYRA® (omadacycline) is a tetracycline-class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms:
Community-Acquired Bacterial Pneumonia (CABP) caused by the following:
Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible isolates), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.
Acute Bacterial Skin and Skin Structure Infections (ABSSSI) caused by the following:
Staphylococcus aureus (methicillin-susceptible and -resistant isolates), Staphylococcus lugdunensis, Streptococcus pyogenes, Streptococcus anginosus grp. (includes S. anginosus, S. intermedius, and S. constellatus), Enterococcus faecalis, Enterobacter cloacae, and Klebsiella pneumoniae.
USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
NUZYRA is contraindicated in patients with known hypersensitivity to omadacycline or tetracycline-class antibacterial drugs, or to any of the excipients.
WARNINGS AND PRECAUTIONS
Mortality imbalance was observed in the CABP clinical trial with eight deaths (2%) occurring in patients treated with NUZYRA compared to four deaths (1%) in patients treated with moxifloxacin. The cause of the mortality imbalance has not been established. All deaths, in both treatment arms, occurred in patients > 65 years of age; most patients had multiple comorbidities. The causes of death varied and included worsening and/or complications of infection and underlying conditions. Closely monitor clinical response to therapy in CABP patients, particularly in those at higher risk for mortality.
The use of NUZYRA during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia.
The use of NUZYRA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth.
Hypersensitivity reactions have been reported with NUZYRA. Life-threatening hypersensitivity (anaphylactic) reactions have been reported with other tetracycline-class antibacterial drugs. NUZYRA is structurally similar to other tetracycline-class antibacterial drugs and is contraindicated in patients with known hypersensitivity to tetracycline-class antibacterial drugs. Discontinue NUZYRA if an allergic reaction occurs.
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs.
NUZYRA is structurally similar to tetracycline-class antibacterial drugs and may have similar adverse reactions. Adverse reactions, including photosensitivity, fixed drug eruption, pseudotumor cerebri, and anti-anabolic action (which has led to increased BUN, azotemia, acidosis, hyperphosphatemia, pancreatitis, and abnormal liver function tests), have been reported for other tetracycline-class antibacterial drugs, and may occur with NUZYRA. Discontinue NUZYRA if any of these adverse reactions are suspected.
Prescribing NUZYRA in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) are nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation.
DRUG INTERACTIONS
Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage while taking NUZYRA.
Absorption of tetracyclines, including NUZYRA is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron containing preparations.
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding is not recommended during treatment with NUZYRA.
Please see Full Prescribing Information for NUZYRA.
References:
- NUZYRA [Prescribing Information]. Paratek Pharmaceuticals, Inc.
- Data on file. Paratek Pharmaceuticals, Inc.