For US Healthcare Professionals only
PRESCRIBINGINFORMATION REQUEST
SAMPLES

NUZYRA is indicated for the treatment of Community-Acquired Bacterial Pneumonia (CABP) and Acute Bacterial Skin and Skin Structure Infections (ABSSSI) in adults caused by select susceptible microorganisms.

EARLY AND EFFECTIVE CLINICAL SUCCESS IN ABSSSI
OASIS-1 study design (N=655)1,2
-
OASIS-1, known as Trial 2 in the NUZYRA Prescribing Information, was a randomized, multicenter, multinational, double-blind, double-dummy trial comparing the noninferiority of 7 to 14 days of NUZYRA to linezolid.
—Types of infections included: wound infection, cellulitis, and major abscess
- NUZYRA was administered in 100 mg doses intravenously every 12 hours for 2 doses followed by 100 mg doses intravenously every 24 hours, with the option to switch to 300 mg orally every 24 hours. Linezolid was administered in 600 mg doses intravenously every 12 hours, with the option to switch to 600 mg orally every 12 hours
Clinical success in ABSSSI: IV to Oral

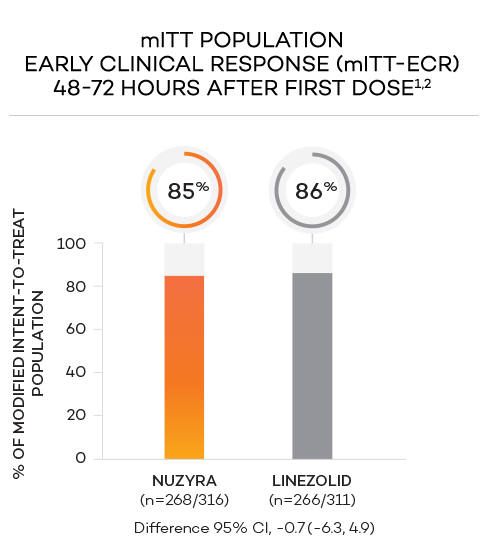
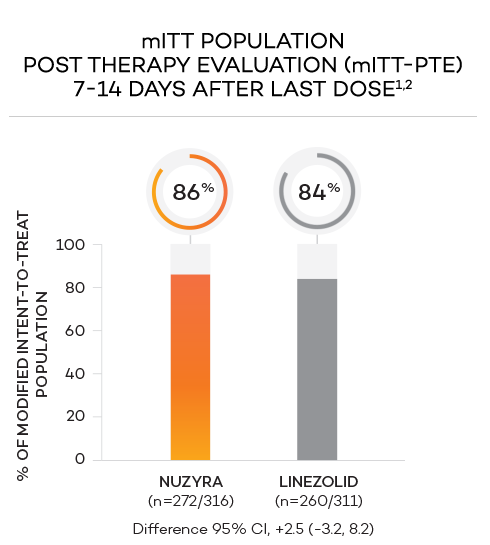
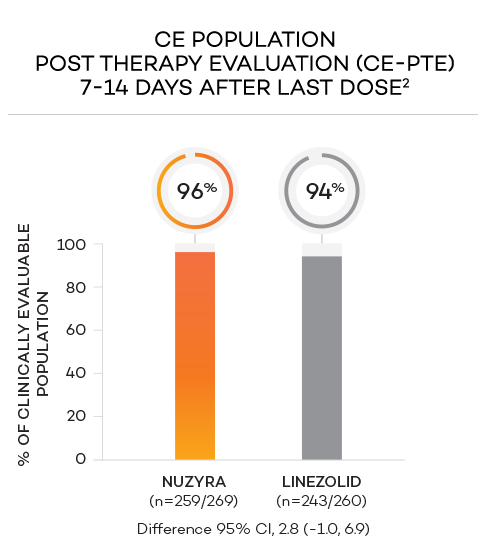
ENDPOINTS1,2
- Primary endpoint at ECR (early clinical response, 48 to 72 hours postinitiation of treatment) in the mITT population was defined as a ≥20% decrease in lesion size, without receiving any rescue antibacterial therapy*
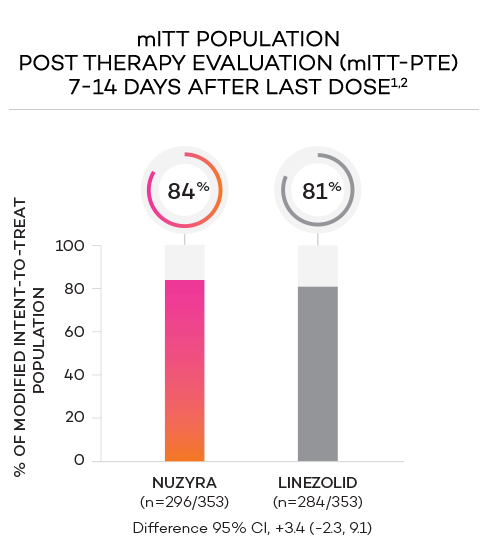
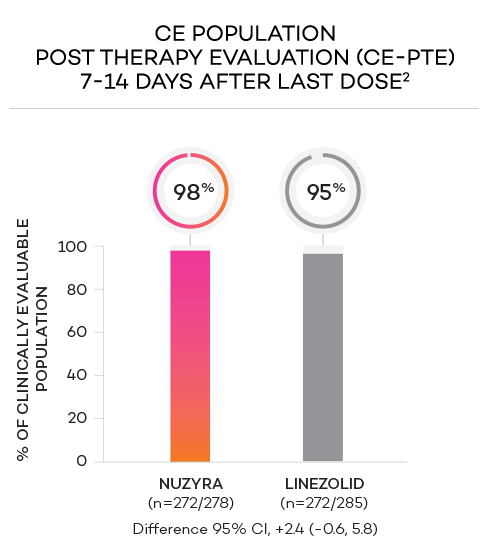
- Secondary endpoint at PTE (post therapy evaluation, 7 to 14 days after last dose) was defined as survival after completion of study treatment without receiving any other antibacterial therapy or unplanned major surgical intervention, and having sufficient resolution of infection such that further antibacterial therapy is not needed, and the infection was considered clinically cured. The secondary endpoint was evaluated in the mITT and in the CE populations
*Reasons for failure included: <20% reduction in lesion size, administration of rescue antibacterial therapy, use of another antibacterial or surgical procedure to treat for lack of efficacy, or death.1
STUDY POPULATIONS1,2
- Modified intent-to-treat (mITT) population was defined as all randomized patients without a sole Gram-negative causative pathogen at screening, due to the lack of Gram-negative activity of linezolid
- Clinically evaluable (CE) population was defined as mITT patients who met inclusion criteria and completed the trial, with a PTE visit 7 to 14 days after the last dose
- Microbiological mITT (micro-mITT) population was defined as all patients in the mITT population who had at least 1 Gram-positive causative pathogen identified at baseline
OASIS-2 study design (N=735)1,2
-
OASIS-2, known as Trial 3 in the NUZYRA PI, was a randomized, multicenter, multinational, double-blind, double-dummy trial comparing the noninferiority of NUZYRA to linezolid
— Types of infections included: wound infection, cellulitis, and major abscess
- NUZYRA was administered in a 450 mg oral dose once a day on Days 1 and 2, followed by 300 mg orally once a day. Linezolid was administered in 600 mg oral doses every 12 hours
Clinical success in ABSSSI: Oral only

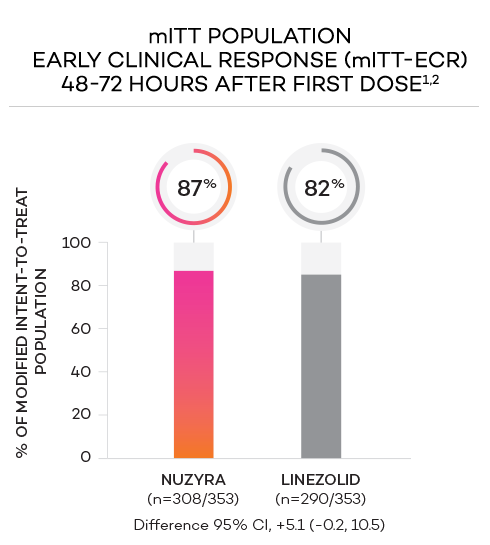
ENDPOINTS1,2
- Primary endpoint at ECR (early clinical response, 48 to 72 hours postinitiation of treatment) in the mITT population was defined as a ≥20% decrease in lesion size, without receiving any rescue antibacterial therapy*
- Secondary endpoint at PTE (post therapy evaluation, 7 to 14 days after last dose) was defined as survival after completion of study treatment without receiving any other antibacterial therapy or unplanned major surgical intervention, and having sufficient resolution of infection such that further antibacterial therapy is not needed, and the infection was considered clinically cured. The secondary endpoint was evaluated in the mITT and in the CE populations
*Reasons for failure included: <20% reduction in lesion size, administration of rescue antibacterial therapy, use of another antibacterial or surgical procedure to treat for lack of efficacy, or death.1
STUDY POPULATIONS1,2
- Modified intent-to-treat (mITT) population was defined as all randomized patients without a sole Gram-negative causative pathogen at screening, due to the lack of Gram-negative activity of linezolid
- Clinically evaluable (CE) population was defined as mITT patients who met inclusion criteria and completed the trial, with a PTE visit 7 to 14 days after the last dose
- Microbiological mITT (micro-mITT) population was defined as all patients in the mITT population who had at least 1 Gram-positive causative pathogen identified at baseline
CLINICAL SUCCESS RATES BY PATHOGEN AT PTE IN ABSSSI1,a
| PATHOGEN | NUZYRA
% (n/N) |
LINEZOLID
% (n/N) |
|---|---|---|
| Staphylococcus aureus | 83% (305/369) | 81% (306/378) |
| Methicillin-susceptible Staphylococcus aureus (MSSA) | 82% (164/201) | 80% (181/226) |
| Methicillin-resistant Staphylococcus aureus (MRSA) | 84% (146/173) | 82% (128/157) |
| Staphylococcus lugdunensis | 91% (10/11) | 67% (2/3) |
| Streptococcus anginosus group | 81% (84/104) | 72% (59/82) |
| Streptococcus pyogenes | 70% (28/40) | 74% (25/34) |
| Enterococcus faecalis | 94% (17/18) | 84% (21/25) |
| Enterobacter cloacae | 79% (11/14) | 82% (9/11) |
| Klebsiella pneumoniae | 73% (8/11) | 55% (6/11) |
aInvestigator’s overall assessment of clinical response at PTE by baseline pathogen in OPTIC (micro-ITT population).
Safety data for NUZYRA
in the treatment of ABSSSI
Is NUZYRA covered by Commercial
and Medicare insurance plans?
Considering NUZYRA
for your patients?
NUZYRA® (omadacycline) is a tetracycline-class antibacterial indicated for the treatment of adult patients with the following infections caused by susceptible microorganisms:
Community-Acquired Bacterial Pneumonia (CABP) caused by the following:
Streptococcus pneumoniae, Staphylococcus aureus (methicillin-susceptible isolates), Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila pneumoniae.
Acute Bacterial Skin and Skin Structure Infections (ABSSSI) caused by the following:
Staphylococcus aureus (methicillin-susceptible and -resistant isolates), Staphylococcus lugdunensis, Streptococcus pyogenes, Streptococcus anginosus grp. (includes S. anginosus, S. intermedius, and S. constellatus), Enterococcus faecalis, Enterobacter cloacae, and Klebsiella pneumoniae.
USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of NUZYRA and other antibacterial drugs, NUZYRA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
NUZYRA is contraindicated in patients with known hypersensitivity to omadacycline or tetracycline-class antibacterial drugs, or to any of the excipients.
WARNINGS AND PRECAUTIONS
Mortality imbalance was observed in the CABP clinical trial with eight deaths (2%) occurring in patients treated with NUZYRA compared to four deaths (1%) in patients treated with moxifloxacin. The cause of the mortality imbalance has not been established. All deaths, in both treatment arms, occurred in patients > 65 years of age; most patients had multiple comorbidities. The causes of death varied and included worsening and/or complications of infection and underlying conditions. Closely monitor clinical response to therapy in CABP patients, particularly in those at higher risk for mortality.
The use of NUZYRA during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia.
The use of NUZYRA during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth.
Hypersensitivity reactions have been reported with NUZYRA. Life-threatening hypersensitivity (anaphylactic) reactions have been reported with other tetracycline-class antibacterial drugs. NUZYRA is structurally similar to other tetracycline-class antibacterial drugs and is contraindicated in patients with known hypersensitivity to tetracycline-class antibacterial drugs. Discontinue NUZYRA if an allergic reaction occurs.
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs.
NUZYRA is structurally similar to tetracycline-class antibacterial drugs and may have similar adverse reactions. Adverse reactions, including photosensitivity, fixed drug eruption, pseudotumor cerebri, and anti-anabolic action (which has led to increased BUN, azotemia, acidosis, hyperphosphatemia, pancreatitis, and abnormal liver function tests), have been reported for other tetracycline-class antibacterial drugs, and may occur with NUZYRA. Discontinue NUZYRA if any of these adverse reactions are suspected.
Prescribing NUZYRA in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥2%) are nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation.
DRUG INTERACTIONS
Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage while taking NUZYRA.
Absorption of tetracyclines, including NUZYRA is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron containing preparations.
USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding is not recommended during treatment with NUZYRA.
Please see Full Prescribing Information for NUZYRA.
References:
- NUZYRA [Prescribing Information]. Paratek Pharmaceuticals, Inc.
- Data on file. Paratek Pharmaceuticals, Inc.